AskBio Brings Parkinson Disease Gene Therapy to Randomized Phase 2 Clinical Trial
In observance of Parkinson's Awareness Month, held annually in April, CGTLive® took a closer look at REGENERATE-PD, a new trial for PD gene therapy AB-1005.

Asklepios BioPharmaceutical (AskBio)’s AB-1005 (AAV2-GDNF), an investigational adeno-associated virus vector serotype 2 (AAV2) gene therapy, is set to be evaluated for the treatment of Parkinson disease (PD) in the phase 2 REGENERATE-PD clinical trial (NCT06285643).1,2 In observance of Parkinson's Awareness Month, an event held annually in April by the patient and clinician community, CGTLive® decided to take a closer look at the phase 2 clinical trial design for this investigational treatment.
AB-1005 is intended to deliver a human glial cell line-derived neurotrophic factor (GDNF) transgene, with the expectation that it will promote survival and morphological differentiation of dopaminergic neurons, the degeneration of which is associated with PD. It is also expected to increase the high-affinity dopamine uptake of these neurons. The gene therapy is administered via a direct neurosurgical injection with MRI-monitored convection enhanced delivery.
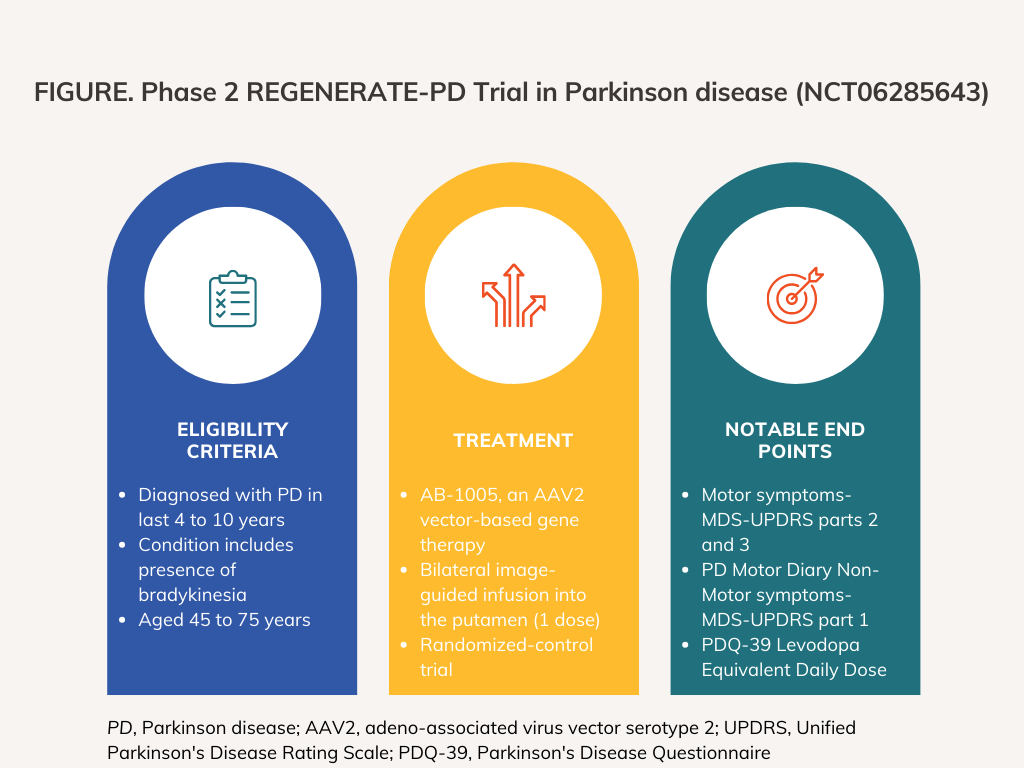
The randomized, double-blind, sham surgery-controlled clinical trial is expected to enroll approximately 87 patients in total and will utilize masking for participants, care providers, investigators, and outcomes assessors. According to the clinicaltrials.gov page, which was published and most recently updated on February 29, 2024, the clinical trial is expected to launch today, April 1, 2024. The study has an expected completion date of November 30, 2027.
REGENERATE-PD, which will utilize parallel assignment for its intervention model, includes 2 arms. Patients in the experimental arm will receive a bilateral image-guided infusion into the putamen of a single dose of AB-1005. On the other hand, patients in the sham comparator arm will undergo a control surgical procedure consisting of bilateral partial burr/twist holes without dural penetration.
REGENERATE-PD's primary end points partially consist of results from parts of the International Parkinson and Movement Disorder Society (MDS)-Unified Parkinson's Disease Rating Scale (UPDRS). In particular, one primary end point consists of the results of Motor symptoms-MDS-UPDRS parts 2 and 3 and another primary end point consists of PD Motor Diary Non-Motor symptoms-MDS-UPDRS part 1. A third primary end point is the Parkinson's Disease Questionnaire (PDQ-39) Levodopa Equivalent Daily Dose. The study’s secondary end points include the change from baseline in the MDS-UPDRS parts 2 and 3 and the PD Motor Diary; the change from baseline in the MDS-UPDRS part 1 and PDQ-39; and the change in Levodopa equivalent daily dose. The timeframe for all primary and secondary end points is 18 months.
The clinical trial will seek to enroll patients who are aged 45 to 75 years old at the time of informed consent. Patients must have been diagnosed with PD during the prior 4 to 10 years with a diagnosis that includes presence of bradykinesia. In addition to the aforementioned requirements, participants’ condition must also include at least 1 of the following: a Modified Hoehn and Yahr stage II-III in the practically defined OFF medication state (at least 12 hours from last dose of antiParkinsonian medications); an MDS-UPDRS Part III score in the practically defined OFF state of 33 to 60; motor fluctuations with at least 2.5 hours of absolute time in the OFF state, averaged over 3 days, which is measured by the PD Motor Diary and assessed at screening, and the initial 2 baseline visits; being on a stable antiParkinsonian medication regimen for at least 4 weeks before screening, with the regimen remaining stable through the baseline timeframe; or being responsive to levopoda, as defined by a change in a modified H&Y stage or MDS-UPDRS Part III score from the OFF state to the ON state after taking the first daily dose of PD medication.
(Click to enlarge)
Patients with a history of significant vascular disease, cardiovascular disease, psychosis, impulse control disorder, malignancy besides treated cutaneous squamous or basal cell carcinomas, or of any medical, genetic, or neurological conditions that could provide an alternative to an idiopathic PD diagnosis, will be excluded from participation in REGENERATE-PD. Patients with current presence of significant cognitive impairment, poorly controlled depression/anxiety, clinically relevant conditions that could compromise surgical suitability and/or patient safety, a contraindication to MRI and/or gadolinium-based contrast agents, as well as those on chronic immunosuppressive therapy, will also be excluded. A history of prior brain surgery of specific types constitutes an additional exclusion criteria, with examples listed as including, but not limited to: deep brain stimulation, pallidotomy-focused ultrasound thalamotomy, or other experimental neurosurgical procedures.
AB-1005 has previously been evaluated in a phase 1b clinical trial (NCT04167540) for patients with PD, which was launched exactly 4 years ago on April 1, 2020. The phase 1b trial, which enrolled and treated 11 participants with AB-1005, remains active, although it is no longer recruiting new patients. Follow-up data on the treated patients will continue to be collected for up to 5 years after their initial treatment.
A few months ago, in January 2024, AskBio announced that the phase 1b trial had met its primary end point in 18-month follow-up data. In terms of safety, there were no serious adverse events attributed to the gene therapy at 18 months posttreatment among the 11 patients and the neurosurgical delivery procedure for the gene therapy, which involved a 1-time bilateral administration directly to the putamen, was deemed well-tolerated. AskBio noted that the study’s goal of achieving greater than 50% target putamen coverage with the gene therapy was surpassed, with a coverage of 63% (±2%) having been achieved. The 11 patients included 6 patients with mild stage PD and 5 patients with moderate stage PD, as assessed by time from diagnosis and baseline severity of symptoms.
“We are encouraged by these early data, which show AB-1005 to be well-tolerated in this study in patients with mild to moderate PD,” Krystof Bankiewicz, MD, PhD, professor and vice chair of research, department of neurological surgery, Ohio State University, and professor emeritus and vice chair of research, University of California – San Francisco, and the cofounder of AskBio subsidiary Brain Neuropathy Bio, said in a January 2024 statement.1 “Although there is still much to learn about this early-stage investigational gene therapy, these first findings will inform our work in this space and have the potential to contribute to the clinical advancement of AB-1005 for the treatment of Parkinson’s disease.”
AskBio originally acquired the AB-1005 program from Brain Neurotherapy Bio (BNB), which merged with AskBio in early 2021.3 Notably, AskBio is also evaluating AB-1005 in a separate phase 1 clinical trial (REGENERATE MSA-101; NCT04680065) for multiple system atrophy-parkinsonian type. In November 2023, the company announced that the first patient was randomly assigned to a cohort in the trial.4