Regeneron Subsidiary Decibel Therapeutics Looks to Assess Gene Therapy for Otoferlin Gene-Mediated Hearing Loss in Phase 1/2 CHORD Clinical Trial
In observance of World Hearing Day, held annually on March 3, CGTLive® took a closer look at the clinical evaluation of hearing loss gene therapy DB-OTO.

Regeneron subsidiary Decibel Therapeutics’ DB-OTO, an investigational adeno-associated virus (AAV) dual-vector-based gene therapy intended to treat otoferlin (OTOF)-related hearing loss, is currently being evaluated in pediatric patients in the phase 1/2 CHORD clinical trial (NCT05788536).1 In observance of World Hearing Day, an event held annually on March 3 that is recognized by the World Health Organization, CGTLive® decided to take a closer look at the clinical evaluation of this ongoing study.2
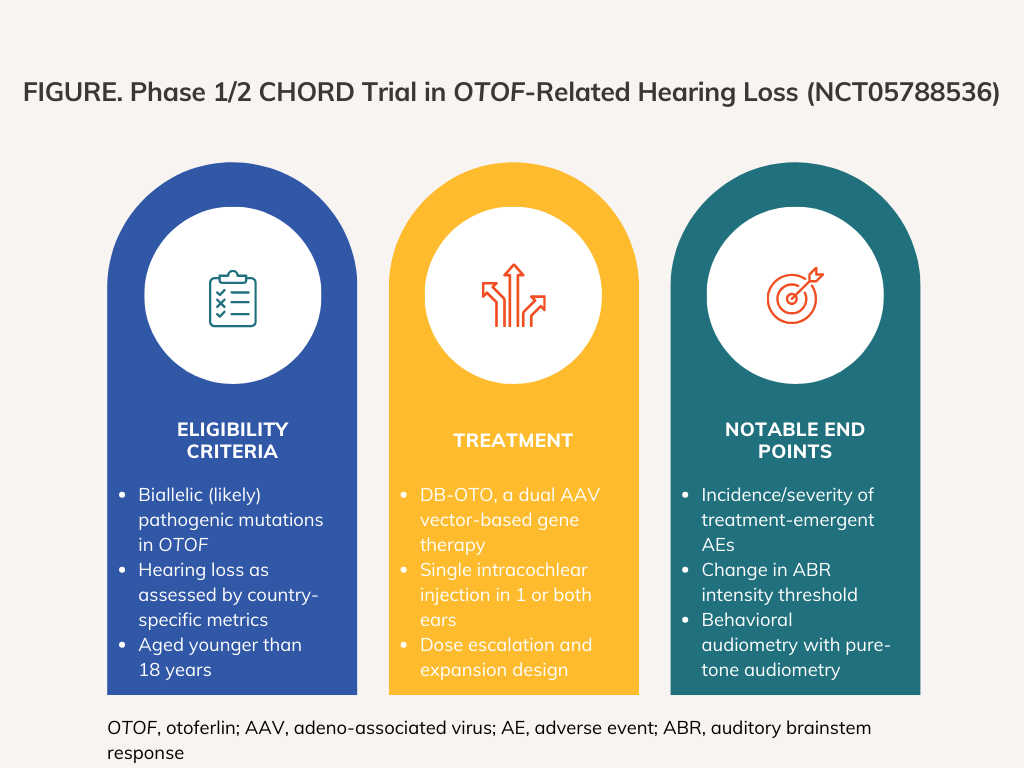
DB-OTO is intended to provide a functional copy of OTOF, is delivered with an injection to the cochlea, and includes a cell-selective Myo15 promoter with the intention of limiting expression of otoferlin, the disease-targeted protein, to cochlear inner hair cells.1,3 The first-in-human, open-label CHORD study, which was launched on May 12, 2023, takes the form of a 2-part dose escalation and dose expansion study and will seek to enroll approximately 22 participants in total (FIGURE). The first part of CHORD, dubbed Part A, will treat patients with a single (unilateral) injection in only 1 ear. Part A includes a lower dose (starting dose) cohort and a higher dose cohort. Based on the safety and efficacy results from Part A, either the lower or higher dose will be selected for use in Part B. Patients in Part B will receive the selected dose in both ears (bilateral injection) during a single surgical session.
According to the clinicaltrials.gov page for CHORD, which was most recently updated on February 7, 2024, the study is actively recruiting patients at locations in California, New York, and Washington in the United States; Cambridge and London in the United Kingdom; and Las Palmas, Madrid, and Pamplona in Spain. An additional US location, Children's Hospital Medical Center in Ohio, is listed as not yet recruiting. The trial has an estimated completion date of August 19, 2030.
(Click to enlarge)
“Spain is an important country within our DB-OTO clinical development strategy, and our trial will leverage the collaborations and natural history work that we have pursued there over the past several years,” Laurence Reid, PhD, the CEO of Decibel, said in a statement made at the time the Spanish Agency of Medicines and Medical Devices cleared the clinical trial application (CTA) for CHORD, in May 2023.4 “Our team believes that DB-OTO could be a transformative treatment for individuals with otoferlin-related hearing loss, and this approval broadens the opportunity to evaluate its potential in infants 2 years of age and younger.”
CHORD’s primary end point is the incidence and severity of treatment-emergent systemic and local adverse events. One of the trial’s secondary end points is the change in intensity threshold, as measured by decibels Hearing Level (dB nHL) across frequency domains, for auditory brainstem response (ABR). The trial’s other secondary end point consists of behavioral audiometry with pure-tone audiometry, which includes the change in intensity thresholds, as measured by dB nHL in the treated ear across frequency domains, the change in speech awareness threshold in the treated ear, and the change in speech reception threshold in the treated ear. The time frame for all end points is 5 years.
CHORD is open to patients younger than 18 years of age who have pathogenic or likely pathogenic mutations in both OTOF alleles. Participants must have parental consent to vaccinations in accordance with their country's pediatric immunization schedule. In the US, an investigator must determine that participants have received minimal benefit from amplification of the ear and that they meet criteria for cochlear implantation in both ears according to the recommended cochlear implant label. US patients 24 months of age or younger must have profound sensorineural hearing loss (SNHL) and confirmed outer hair cell function. US participants older than 24 months of age must meet the same criteria as younger patients but also have behavioral open-set word recognition scores of less than 30% and a cochlear microphonic in the ear(s) that would receive DB-OTO. In the UK and Spain, participants must have no ABR neural signal in response to a click stimulus less than or equal to 85 dB nHL and presence of otoacoustic emissions in the ear(s) that would receive DB-OTO. Participants in the UK and Spain that are older than 24 months of age must additionally have a cochlear microphonic in the ear(s) that would receive DB-OTO.
Patients with a history of other permanent or untreatable hearing loss conditions; prior or current cochlear implants in the ear(s) to be injected with DB-OTO; a history of a risk factor or risk factors for auditory neuropathy not caused by OTOF mutations; a history of malignancies or meningitis; or surgical anatomy that would prevent the planned surgical approach will be excluded from participation. Participants must also not have previously been treated with any gene therapy; must have no clinically significant laboratory findings at screening; and must not show evidence that their hearing loss depends on body temperature. The clinicaltrials.gov page indicates that additional inclusion and exclusion criteria might apply per protocol.
Manohar Bance, MBChB, MSc, FRCSC

The CTA in Spain was the most recent regulatory clearance for CHORD; Decibel previously received clearance of a CTA for DB-OTO from the United Kingdom’s Medicines and Healthcare products Regulatory Agency in January 2023 and clearance of an investigational new drug application for the gene therapy from the FDA in October 2022.3,5 Regeneron announced in October 2023 that the first child in CHORD had been treated and showed some improvements in auditory responses.1 The child, who was stated to be less than 2 years of age in the announcement, was treated with DB-OTO in 1 ear. Investigators found that the child experienced improvements in ABR and pure tone audiometry through 6 weeks of follow-up as compared to baseline.
"We are still in the early phases of understanding the effectiveness of these therapies," Manohar Bance, MBChB, MSc, FRCSC, an ear surgeon and principal trial investigator at Cambridge University Hospitals NHS Foundation Trust in the UK, told CGTLive in March 2024. "However, our week 6 and week 12 DB-OTO results for hearing improvement for our first child, who was 10 months old and one of the youngest in the world to be treated, in terms of hearing sound have been very impressive. The week 12 CHORD trial results were presented at the Association for Research in Otolaryngology Meeting in February 2024. We still have to follow how this translates into understanding speech itself as the child grows older, but we look forward to monitoring their progress as well as the other patients we are enrolling. This lends momentum to the gene therapy movement for genetic hearing loss, and we all hope is the beginning of an era of reversing hearing loss rather than bypassing it."
“The children who are being enrolled in CHORD are often born with profound hearing loss due to mutations in a single gene, OTOF, which essentially turns off their auditory circuits,” Bance said earlier in an October 2023 press release from Regeneron.1 “Cochlear implants are the current standard of care but are unable to replicate the full complexity and range of sound. With these very preliminary DB-OTO results, we now have encouraging evidence that this gene therapy may be able to help turn these auditory circuits back on. We look forward to following this child and others further to determine if DB-OTO gene therapy can restore clinically meaningful hearing as they are learning to interact with the world.”